Home
electronic Patient Benefit Index
(10-item short version)
ePBI-10
Quick and easy assessment of
therapy benefit
What is the ePBI-10?
The ePBI-10 is a validated and shortened version of the ePBI and comprises only 10 questions instead of 25. Both instruments were developed for dermatology patients and their physicians and are based on the concept of the Patient Benefit Index (PBI).
Similar to the ePBI, the ePBI-10 can be used in clinical research to record patient-relevant aspects of therapy and its benefits. In addition, the ePBI-10 can also be used in clinical practice for shared decision-making when there is not enough time to use the more comprehensive ePBI. However, the ePBI with all 25 questions enables a more comprehensive benefit assessment and is therefore preferable in practice.
Validation of questionnaire
paper version: yes digital version: yes
How to use the ePBI-10
Before the beginning of treatment, the patient completes a questionnaire with 10 items on their treatment goals (PNQ: Patient Needs Questionnaire) and indicates how important these are to them on a 5-point scale from "not at all" to " very" or whether they do not apply to them. After starting treatment, the patient completes the Patient Benefit Questionnaire (PBQ) with 10 items and indicates the extent to which the treatment has helped them achieve the previously listed goals or whether these did not apply to them. Once again, this is rated on a 5-point scale from "not at all" to "very". Finally, the overall benefit of the therapy is calculated from the answers given.
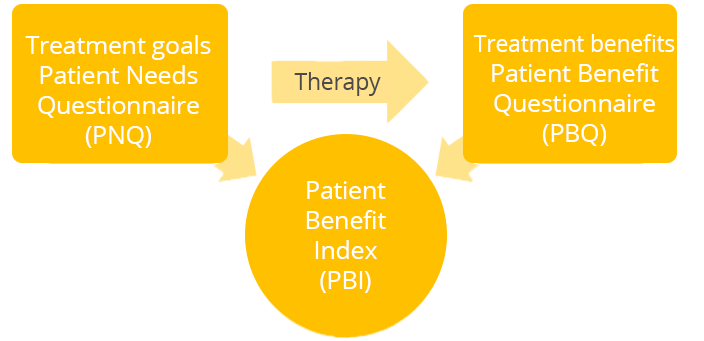
The index range is between 0 (no patient-relevant benefit) and 4 (maximum patient-relevant benefit).
Below is an example of the PBI calculation process in four steps.
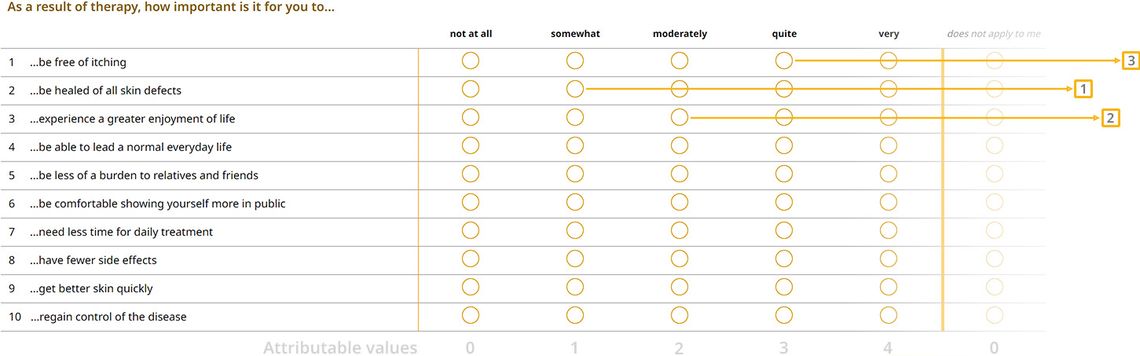
1) Importance of treatment goals
With the help of the following questions, we’d like to know how important the goals mentioned below are to you personally in the current treatment of your skin disease.
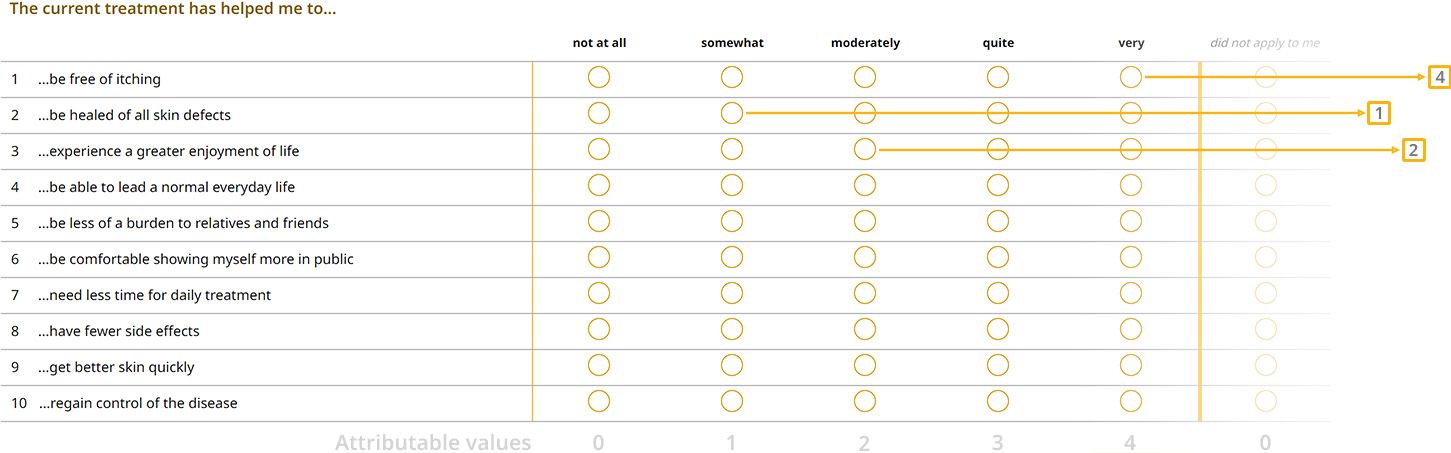
2) Treatment benefits
When the treatment began, you indicated in a questionnaire how important various goals were in the treatment of your skin disease.
3) Calculation PBI
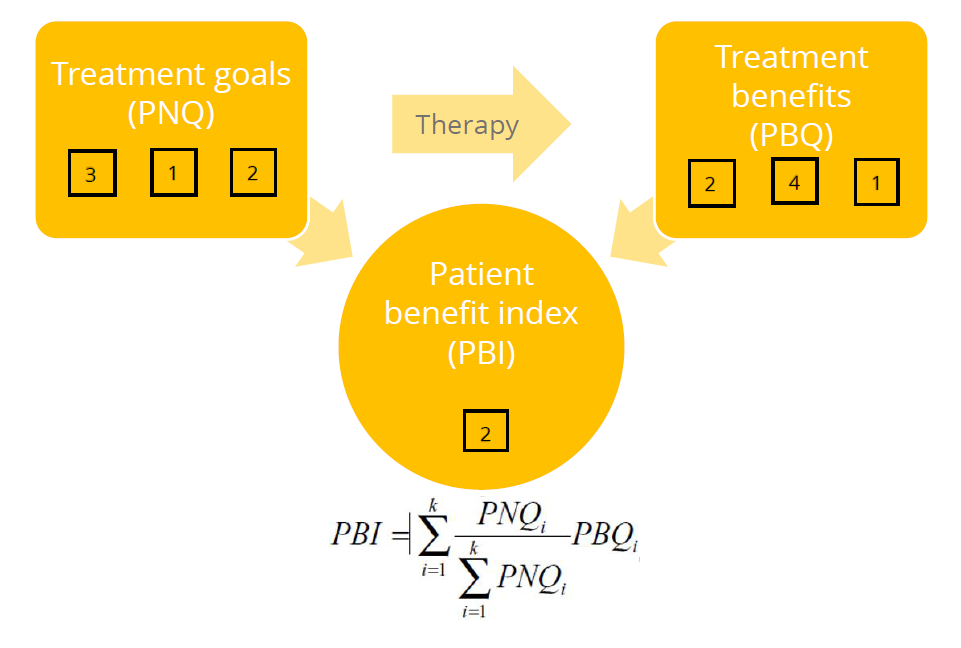
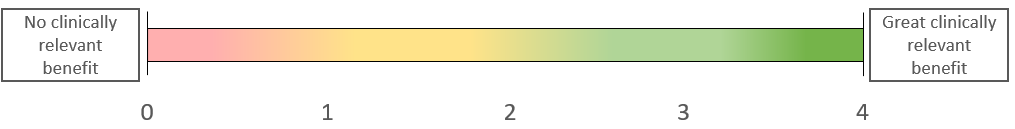
4) View of results (example)
The following versions of the PBI-10 are available
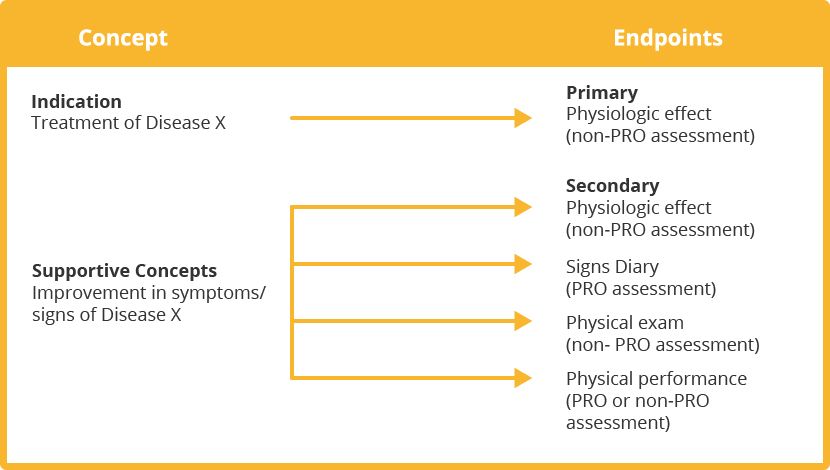
Endpoint Model: Treatment of Disease X
Food and Drug Administration. Guidance for industry – Patient-Reported Outcome Measures: Use in medical product development to support labeling claims. US Food and Drug administration website, 2009 [cited 2017]
About Patient Reported Outcomes
“A PRO is any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.”
“Use of a PRO instrument is advised when measuring a concept best known by the patient or best measured from the patient perspective. […] PRO instrument adequacy depends on its role and relationships with other clinical trial endpoints as depicted in the endpoint model.”
Features
USER-FRIENDLY
The intuitive and interactive design of the ePBI-10 ensures easy understanding of all questions, whether for physicians or patients.
QUICK
Save time with our online tool. Fill in the questionnaire and your ePBI-10 score will be calculated automatically.
RESULT FEEDBACK
On DermaValue, you not only get your PBI-10 value, but also learn what it means in terms of your therapy benefit.
MULTILINGUAL
The PBI-10 is an established tool and available in several languages. The use of the respective first language reduces language barriers and supports the understanding between physician and patient.
ACCESSIBLE FROM ALL DEVICES
With DermaValue, your ePBI-10 is always just a few clicks away. The application is available for PCs, tablets, smartphones, and other devices.
ENVIRONMENTALLY FRIENDLY
Take care of the environment and save your patient data on the online platform or as PDF files for yourself and your physicians.
References
Blome C, von Stülpnagel CC, Augustin M, Mrowietz U, Reich K, Muehlan H, Kirsten N, Langenbruch AK, Sorbe C, Klein TM. Measuring patient-relevant benefits in the treatment of psoriasis with the Patient Benefit Index: development and preliminary validation of a 10-item short form. Br J Dermatol. 2022 Oct;187(4):588-589. doi: 10.1111/bjd.21593 . Epub 2022 May 31. PMID: 35383904 .
Our other tools
Our partners







